A concerted assault on myeloproliferative neoplasms
The Ludwig Oxford Branch launched this year a research program focused on myeloproliferative neoplasms (MPNs), a family of typically slow-growing blood cancers that in some patients transform into aggressive leukemias. Known as blast-phase MPN (BP-MPN), this transformation of the disease has an associated life expectancy of 6-12 months. All three types of MPN—essential thrombocythemia (ET), polycythemia vera (PV) and low-risk myelofibrosis—are in most cases indolent cancers, accompanied by some symptoms but only a modest reduction in life expectancy. About one in three MPN patients, however, develops a high-risk type of myelofibrosis with a median survival of 5-7 years. About 20% of the latter, and 1-5% of ET and PV patients, progress to BP-MPN, for which there is currently no treatment that improves outcomes.
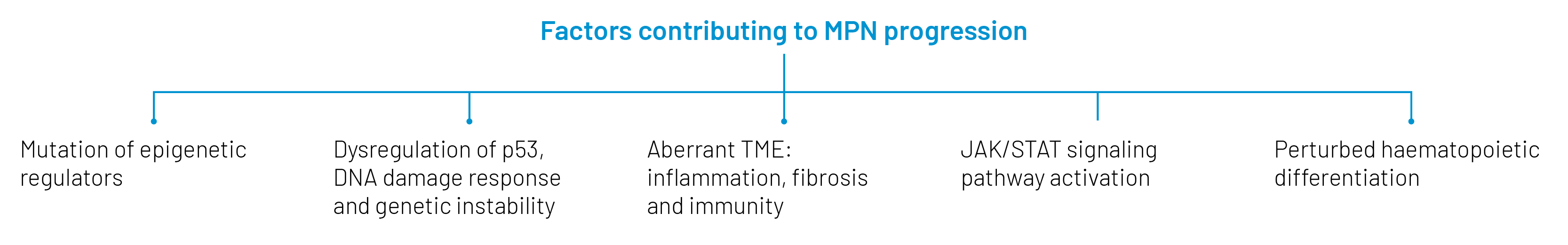
MPNs are driven by mutations in hematopoietic (blood-forming) stem cells to three genes whose products participate in signaling through the thrombopoietin receptor (TPO-R) via the JAK-STAT pathway: JAK2 (the famous JAK2V617F mutation), calreticulin (CALR) and TPO-R (MPL). Though drugs that inhibit JAK2 control MPN symptoms in most patients, they do not stall the disease or prevent progression to BP-MPN and have little efficacy against the secondary leukemia. That escalation is fueled by mutations to the tumor suppressor TP53, epigenetic regulators (like EZH2, ASXL1)—which chemically tag DNA and histones to regulate gene expression—and transcription factors expressed by myeloid cells (such as RUNX1). Gene copy number alterations also drive that transformation and could offer clues to the development of therapies for BP-MPN.
The development of such therapies is a critical unmet need in clinical oncology. So is a better understanding of risk factors for the progression of MPNs to BP-MPN, about which precious little is currently known. Identification of those risk factors would not only improve monitoring of patients at high risk for secondary leukemia, but might enable the development of new therapies for BP-MPN and interventions to capture such cases early and perhaps even prevent disease progression.
The MPN Program at Ludwig Oxford, which will be conducted in collaboration with researchers at the MRC Weatherall Institute of Molecular Medicine (MRC WIMM) and the Radcliffe Department of Medicine at the University of Oxford, addresses these needs. It aims to identify mechanisms and related biomarkers of disease progression and to use such insights to develop therapeutic strategies to treat and prevent BP-MPN. This requires a defined spectrum of expertise and the development of research tools, disease models and patient cohorts—all of which are available or can be developed at the Oxford Branch and MRC WIMM. The initiative especially benefits from a critical mass of scientific talent in epigenetics, cancer plasticity, signaling and p53 tumor suppressor mechanisms already assembled at the Oxford Branch. Further, its translational objectives are aided by the presence in its leadership of clinicians experienced in the care of MPN patients.
Overseen by four program leads—physician-researchers Ludwig Oxford’s Bethan Psaila, Stefan Constantinescu and MRC WIMM’s Adam Mead along with Ludwig Oxford Director Xin Lu—the MPN initiative has three arms that pull together the required scientific expertise. One is dedicated to exploring the epigenetics, molecular biology and aberrant signaling underlying MPN progression to acute leukemia and testing the veracity of its mechanistic models, which will inform translational efforts. A second arm is dedicated to the discovery of biomarkers and the development and validation of translational tools and technologies—ranging from imaging to machine learning to liquid biopsies and epigenetic mapping—for their efficient detection. The third focuses on obtaining samples from large cohorts of patients with myelofibrosis and the development of human iPSC-based xenograft and human organoid models to vet the clinical hypotheses generated by the project.
The Link thinks science just got even more exciting at the Oxford Branch.