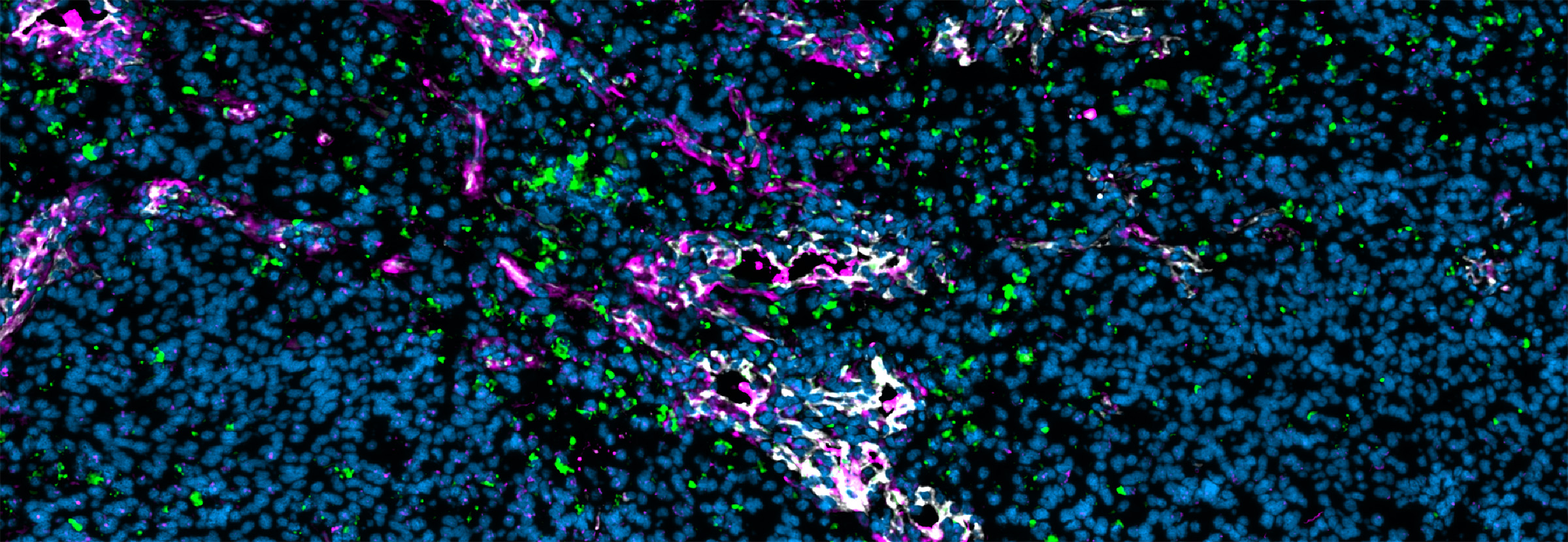
Primary and metastatic brain tumors show considerable variation in their immune microenvironments, and these differences are of significant relevance to the success of novel therapeutic strategies now under development. The tumor vasculature, a key component of that microenvironment, serves as an active gatekeeper of immune cell infiltration and a mediator of immunosuppression and drug resistance. Researchers led by Ludwig Lausanne’s Leire Bejarano and Johanna Joyce reported in an Immunity paper published online in March (and as the cover article of the April issue) an in-depth, comparative analysis of individual cells—focusing on endothelial and mural cells—that compose the blood-brain barrier across healthy brain tissue, IDH-mutant low grade gliomas, high-grade glioblastomas (GBMs), and brain metastases arising from various cancers. Integrating immunofluorescence microscopy and spatial analyses with single-cell transcriptomics and bulk RNA sequencing, the researchers found that alterations in tumor-feeding blood vessels are far more pronounced in metastatic brain tumors than in gliomas, with the former showing higher permeability and interactions with distinct immune cell populations. Within primary brain tumors, GBMs show more pathological alterations in their vasculature than do low-grade gliomas. The high-resolution vascular atlas generated by this study lays the foundation for the development of novel vascular- and immune-targeting therapies for brain tumors.
Single-cell atlas of endothelial and mural cells across primary and metastatic brain tumor
Immunity, 2025 March 18