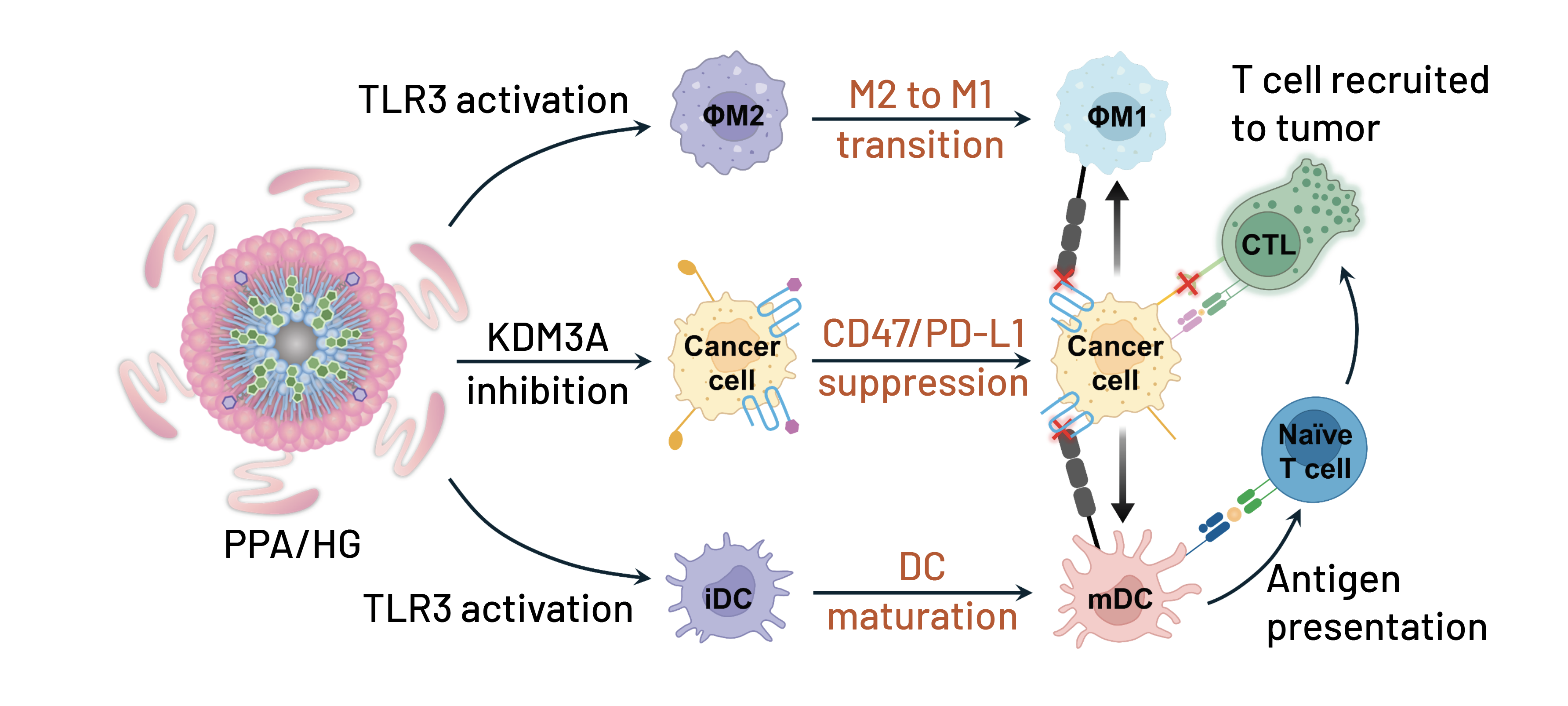
Researchers led by Ludwig Chicago’s Wenbin Lin reported in a couple of papers their design and preclinical evaluation of two types of nanotechnology with distinct mechanisms of action and potential as cancer therapies. A February paper in ACS Nano led by Wenbin and Jing Liu described a core-shell nanoparticle, PPA/HG, for immunotherapy. Wenbin, Jing and colleagues showed in their study that PPA/HG—comprising polyinosinic: polycytidylic acid (PPA) in the core and a cholesterol-conjugated prodrug of 3-(hydroxyolinoyl)glycine (HG) on the shell—persists stably in the blood and accumulates in tumors, where it releases its active ingredients. HG suppresses the expression by cancer cells of CD47—which would otherwise transmit a “don’t eat me” signal to macrophages—as well as PD-L1, which inhibits CD8+ T cell targeting. PPA, meanwhile, activates toll-like receptor 3, which flips tumor-associated macrophages from a pro-tumor state to an anti-tumor state and drives the maturation of dendritic cells, which boost CD8+ T cell responses to tumors. Together, the two drive CD8+ T cell infiltration and deplete regulatory T cells, which suppress immune responses, in tumors. Systemic administration of PPA/HG inhibited tumor progression in mouse models of triple-negative breast cancer and pancreatic ductal adenocarcinoma with minimal side effects.
The other study, published in January, reported in Advanced Science the design and assessment of Hafnium (Hf)-based nanoscale metal-organic layers (MOLs) for cancer therapy. Hf-based MOLs enhance the therapeutic effects of tissue-penetrating X-rays by significantly amplifying the generation of hydroxyl radical and singlet oxygen species, which damage DNA and induce cell death. But their therapeutic efficacy is often compromised by low oxygen levels in tumors and the short half-lives of reactive oxygen species. In this paper, Wenbin and his colleagues detailed their design, production and evaluation of a MOL that generates superoxide anion (O2−) and releases nitrogen oxide (NO) in response to low-dose radiation. Sustained release of NO not only relieves intratumoral hypoxia to reduce resistance to radiotherapy but also reacts with O2− to form long-lived and highly cytotoxic peroxynitrite, which causes double-strand breaks in nuclear DNA. The researchers show that this MOL (named SNAP-MOL), when combined with radiotherapy, inhibits tumor growth in mouse models of colorectal and triple-negative breast cancer.
Nanoparticle-Mediated Toll-Like Receptor Activation and Dual Immune Checkpoint Downregulation for Potent Cancer Immunotherapy
ACS Nano, 2025 February 26
Nitric Oxide-Releasing Nanoscale Metal-Organic Layer Overcomes Hypoxia and Reactive Oxygen Species Diffusion Barriers to Enhance Cancer Radiotherapy
Advanced Science, 2025 January 1