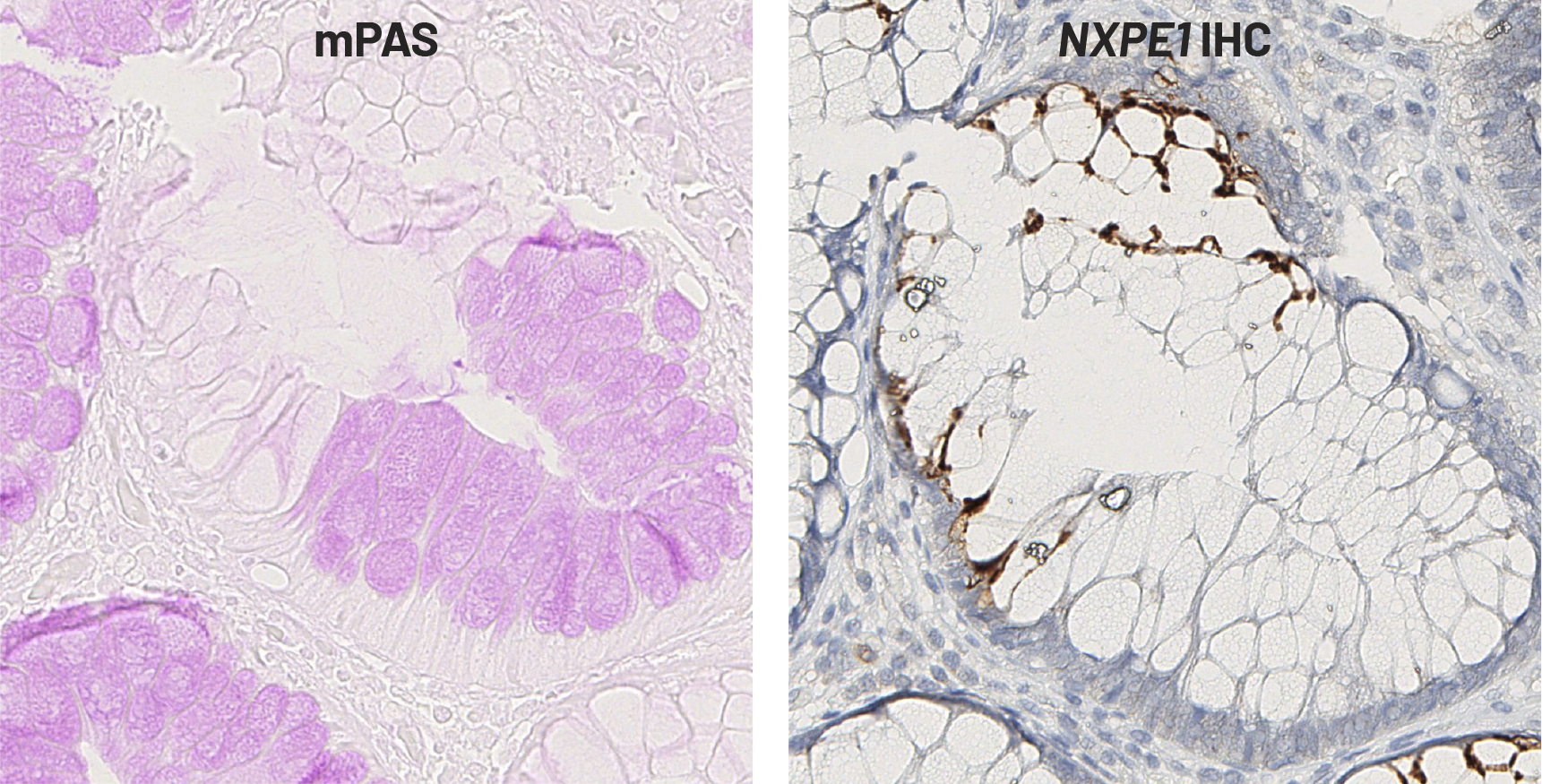
Mild periodic acid Schiff staining (mPAS) has been used for over five decades to characterize sialoglycans (i.e. sialoglycoproteins and glycolipids) in tissue specimens, used for studies ranging from investigations of cell adhesion to immunology to cancer biology. These studies reveal striking variation in mPAS staining in human colorectal tissue but the cause of this variation was unknown. To find that cause, researchers led by Ludwig Johns Hopkins’ Bum Seok Lee, Kenneth Kinzler and Nicolas Wyhs used haplotypes—stretches of DNA inherited intact from one parent—derived from whole genome sequencing to explore chromosomal regions associated with differences in mPAS staining. They reported in a May issue of Nature Communications that a haplotype on chromosome 11 is associated with such differences in colorectal tissue. The researchers found that NXPE1 is the gene within this haplotype responsible for the observed variations, and that altering a single nucleotide polymorphism in its promoter influences its expression and changes modified sialic acid levels. They also showed that the recombinant product of NXPE1 is able to acetylate oxygens on sialic acids in vitro and that this chemical modification disrupts the chemistry of mPAS staining, solving a 50-year old mystery of microscopy and pathology. The researchers present NXPE1 as a prototype of a new family of sialic acid O-acetylation-modifying enzymes and as the gene responsible for differences in colon mPAS staining.
NXPE1 alters the sialoglycome by acetylating sialic acids in the human colon
Nature Communications, 2025 May 27