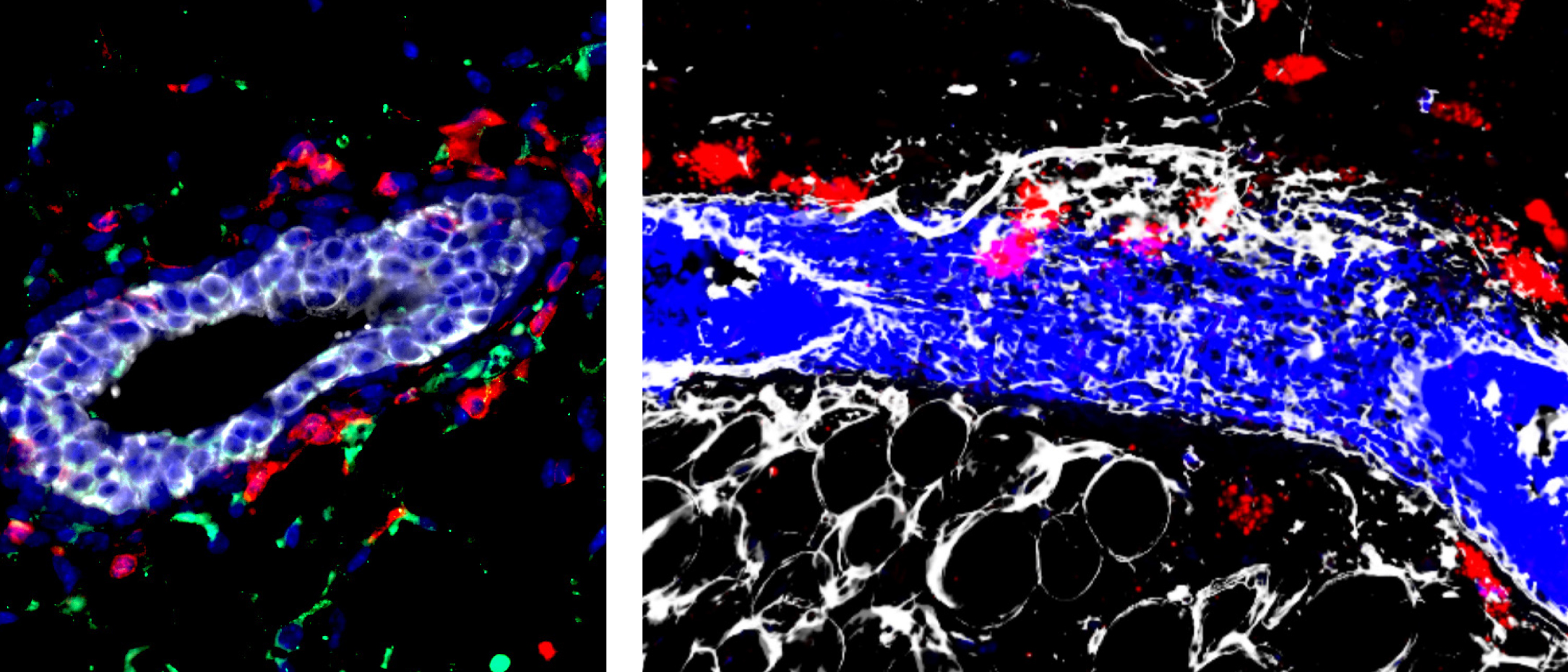
To establish tumors, tumor initiating cells (TICs)—a.k.a. cancer stem cells—in the breast and elsewhere exploit many of the same intrinsic factors that regulate normal stem cells. But considerably less is known about the microenvironmental cells and extrinsic factors that regulate mammary stem cells (MaSCs) and TICs in the breast. In a May issue of Nature Communications, researchers led by Ludwig Princeton’s Yibin Kang and Eunmi Lee identified ductal macrophages expressing the chemokine receptor CXCR4 as a key niche population in normal mammary ducts. They report that these CXCR4+ macrophages promote the regenerative activity of the mammary duct’s contractile basal cells in response to the chemoattractant CXCL12 produced by its milk-producing luminal cells. The same CXCR4+ niche macrophages regulate the tumor-initiating activity of various breast cancer subtypes by enhancing cancer stem cell survival and tumor-forming capacity. They also nurture an immunosuppressive niche by expressing ALDH1a2, an enzyme whose product induces the differentiation of immunosuppressive regulatory T cells. The genetic depletion or pharmacological targeting of the CXCL12–CXCR4 signaling axis or ALDH1a2 inhibit tumor initiation, progression and metastasis by reducing the number and activity of TICs and Tregs while boosting the numbers of cytotoxic CD8+ T cells, which can kill cancer cells. Yibin, Eunmi and colleagues also show that a CXCR4+ niche macrophage gene signature correlates with poor prognosis in human breast cancer.
CXCR4+ mammary gland macrophageal niche promotes tumor initiating cell activity and immune suppression during tumorigenesis
Nature Communications, 2025 May 25