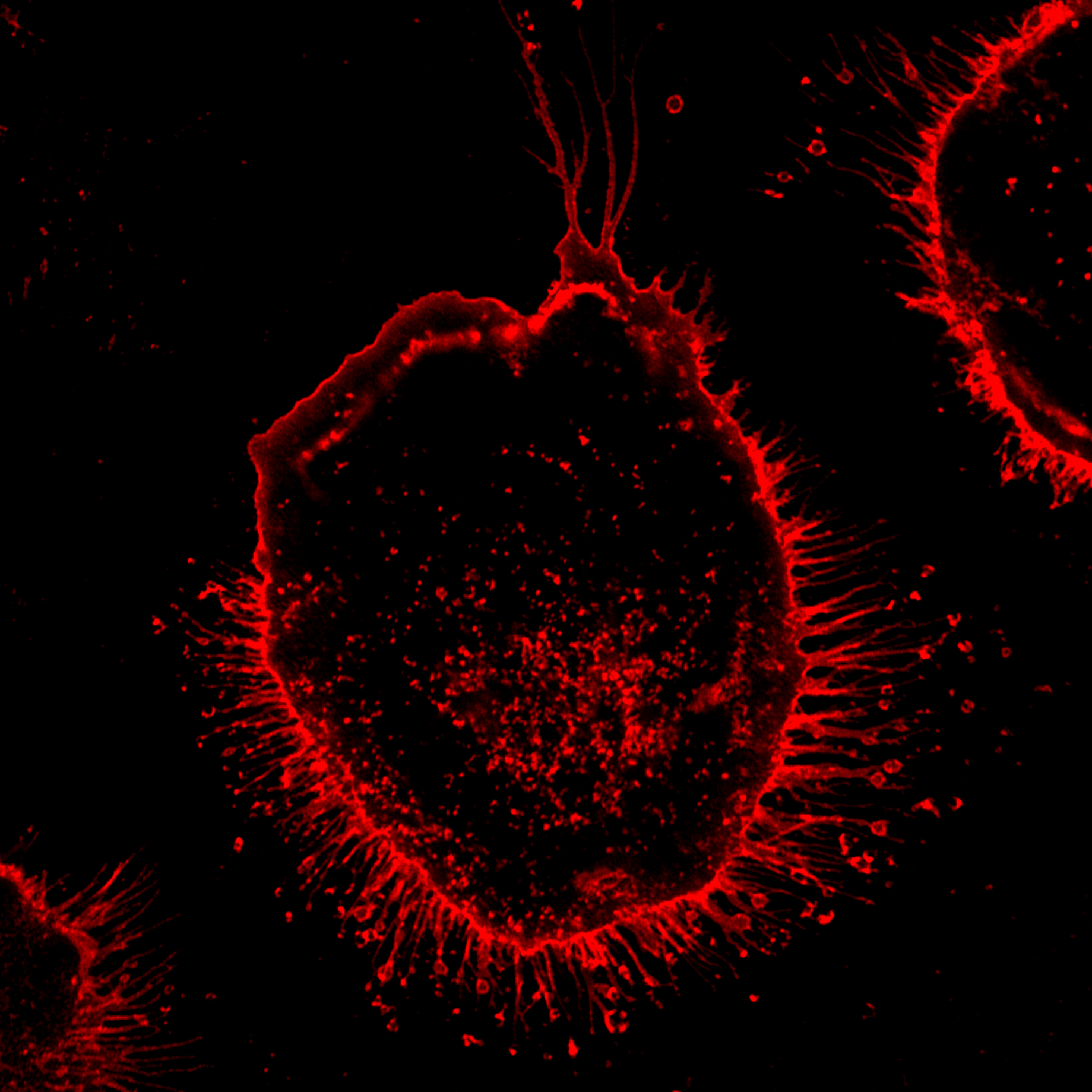
Recent studies have revealed that macrophages can transfer their own calreticulin (CALR) proteins to exposed asialoglycans on sick, unwanted or dying cells to direct their removal by phagocytosis, a process known as programmed cell removal (PrCR). Researchers co-led by Ludwig Stanford’s Allison Banuelos and Irving Weissman explored the mechanisms by which CALR is processed, secreted and binds to its targets on cells. They reported in a May publication in PNAS that upon secretion by lipopolysaccharide-activated macrophages, CALR is cleaved—likely by cathepsins—at one end (the carboxy-terminal) and that this truncated CALR decorates the surface of target cells, serving as an “eat me” signal for macrophage phagocytosis. They also show that macrophages secrete neuraminidases, which clip off sialic acid residues on the cell surface and so prime their targets for binding by the cleaved CALR. A better understanding of these mechanisms of PrCR could inform the design of therapies to better recruit macrophages to the removal of cancerous and otherwise pathogenic cells. The authors note, however, that while their study describes how macrophages paint their targets for destruction, how they recognize which cells to target remains a mystery.
Macrophages release neuraminidase and cleaved calreticulin for programmed cell removal
PNAS, 2025 May 21